
Using SmartSkin in clean room environments for pharmaceutical manufacturing
Cleanroom Classifications
Manufacturing of sterile products takes place under highly controlled conditions to ensure absence of contamination. Introducing any material into a sterile manufacturing environment poses risks for microbial contamination, therefore critical control measures are taken into consideration to maintain sterility and prevent contamination. To mitigate contamination risks Environmental, Personnel, Materials and Equipment control measures are implemented. Environmental controls include use of specific and dedicated cleanrooms, regular environmental monitoring and strict cleaning and disinfection procedures.
Cleanrooms are controlled environments used to minimize contamination during the manufacturing of sterile products. They are classified into different grades based on the level of cleanliness required for the operation or step along the process. Different facilities and technologies utilized for parenteral products manufacturing may require different cleanroom classification.
Cleanrooms ensure the safety, purity, and quality of sterile pharmaceutical products by controlling contamination from particles, microorganisms, and pyrogens. These environments ensure that specified levels of cleanliness are maintained for product quality from formulation to packaging and allow companies to market their products in accordance with global regulatory compliance standards.
Good Manufacturing Practices (GMP) establish four grades of cleanrooms for sterile product manufacturing. ISO classifications and GMP cleanroom grades are closely related but serve different purposes in cleanroom standards. Around the globe, there are varying classifications of GMP Cleanroom requirements where some may adhere to ISO standards and others to their equivalent A,B,C and D facility grades as shown in the graphic below.

Maintaining standards across all steps of the process
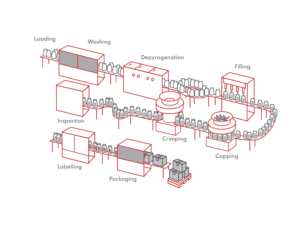
SmartSkin Technologies assists pharma manufacturers in optimizing production with the combination of hardware and software that captures the experience of your product on the line. Used in parenteral filling and packaging processes, our Container Twins can be customized to match different container sizes and formats (such as vials, syringes, cartridges, pen injectors etc,) and are generally put on conveyor lines to measure levels of pressure, and shock along with tilt, spin and other interactions that may impact the quality of product in its container closure system. They have a variety of uses including auditing the lines for adequate equipment set-up, troubleshooting during occurrence of defects, glass breakage, identifying causes of rejection, verifying and assisting in equipment set up prior to production, new equipment design and installation, technology transfer at new sites etc. The SmartSkin system can be used from the washing to filling, capping and vial inspection stages of the production process.
We are therefore often asked about how SmartSkin fares in Cleanroom environments and allows manufacturers to maintain GMP compliance. So how can SmartSkin be utilized in controlled environments?

Practical Applications
As a result of its troubleshooting capabilities SmartSkin’s solutions are typically used during changeovers, downtimes and maintenance. They are often dedicated to a cleanroom to minimize transfer between sterile and non-sterile environments. All materials provided in SmartSkin kits are wiped with disinfecting agents and can pass through airlock or pass-through hatches if needed following approved SOPs. The Container Twins can therefore be used:
- Alone or alongside empty, water-filled containers through filling processes,
- Alongside sealed containers for packaging processes.
The Container Twins can also be VHP Sterilized if necessary and SmartSkin also can make customizations with contact charging designs, and storage cases to minimize potential contamination risks.
The following graphic shows which stages of the process can use SmartSkin container twins during downtimes.
Key Takeaways
The exponential growth of the pharmaceutical industry and the ever rising demand of products significantly impacts parenteral manufacturing processes. In addition to the tensions of keeping up with high demands, pharma manufacturers must also continue to evolve their product integrity practices to match GMP and Annex 1 requirements. While SmartSkin systems assist in maintaining these standards, they must also be easy to use in sterile environments to prevent contaminating products themselves. The technology can therefore be proficiently cleaned to use in Grade D,C,B environments. When it comes to Grade A environments, line technicians can still run a batch of Container Twins but specifically during downtimes and stoppages to monitor equipment conditions.
By identifying points of pressure or validating the line set up, SmartSkin helps proactively prevent defects and reject rates, making it easier to protect container integrity and comply with regulatory standards.


