
Leveraging container twin technology in pharmaceutical manufacturing
In the quality and regulation-driven world of pharmaceutical manufacturing, maintaining optimal process control is crucial. SmartSkin’s container twin technology is being leveraged to quickly and easily obtain real-time container handling data across filling and packaging processes – directly from the container’s perspective.
With instant, actionable insights into line performance, manufacturing teams can:
- identify high-risk areas for breakage, scratching, and other glass defects,
- confirm that issues are resolved after troubleshooting and maintenance activities,
- ensure proper equipment setup before every run for proactive risk mitigation, and
- support continuous improvement activities.
How it works
SmartSkin’s container twins are sensors designed to match the exact shape and size of any production container; from vials, ampoules and bottles to syringes, cartridges, and auto-injector pens.
They are placed directly in the process alongside empty or water-filled containers and with hundreds of external and internal sensors in each device, can quantify your containers’ experience on the line.

Vial container twin on a conveyor
The real-time data collected and analyzed includes:
- Sidewall pressure– under the red film on each device lies hundreds of pressure sensors that quantify the pressure exerted on the body of your containers and pinpoint exactly where those forces are being applied.
- Shock – both horizontal and vertical shock measurements allow you to identify areas of high or frequent impacts that weaken the glass and can result in breakage and defects both on the spot, and further downstream in the process or supply chain.
- Spin, tilt and rotation – assess the spin and rotation in degrees and rpm to identify areas of excess motion to troubleshoot labelling issues, splashing, and other material handling issues.
- Top load and seal tightness– our specialty Seal Tightness container twins allow you to assess the force being applied by each capping head and monitor the seal tightness over time after crimping to improve your seal assurance strategy.
With all data visualized with synced video footage of the container twin on the line, you can instantly pinpoint high-risk areas for damage and improper equipment setup.
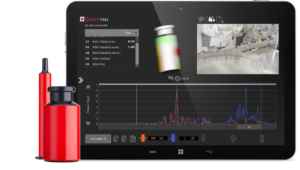
Vial and syringe container twins.
Common issues identified
For pharma manufacturers, leveraging the technology when faced with damage and defects allows them to accelerate root cause investigations by pinpointing damaging areas on the line. Common equipment issues identified include:
- Misaligned guide rails
- Excess pressure at starwheel transitions
- Excess shock at accumulation tables
- High frictive contact during mass transfer
- Accelerated part wear
- Equipment design issues
- Inconsistent top load force between capping heads
- High shock at transition plates
- Sub-optimal equipment settings, ex. speed variation between sections
Where quick adjustments are possible, they are made on the spot and the container twin is run again to verify that the issue was resolved. Design issues and more complex challenges are scheduled into upcoming maintenance or prioritized as part of continuous improvement projects.
After critical issues are resolved, the latest software also allows control limits to be set for control points (high-risk areas with a high potential for variability). Instant alerts indicate when pressure, shock, or motion deviates beyond the acceptable range. This enables technicians to quickly verify proper equipment setup before every run to proactively mitigate risks and optimize line performance.
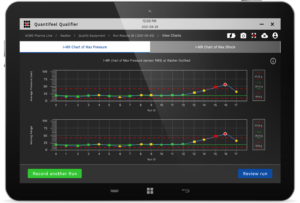
SmartSkin’s latest software with control charting capabilities.
Applications
Applications in pharmaceutical manufacturing range from on-the-spot troubleshooting to routine use between batches as part of enhanced QRM, and project-based initiatives supporting CAPA, continuous improvement, preventative maintenance and new equipment commissioning activities.
Enhance QRM strategies
- Quantitative container handling data enables data-driven decision-making and risk assessments.
- High-risk areas for damage can be instantly identified and goals can be set to support continuous improvement initiatives.
- Routine monitoring of critical areas reduces the frequency of glass damage, defect, and rejects, and reduces product loss; reducing both production costs and the risk of supply shortages.
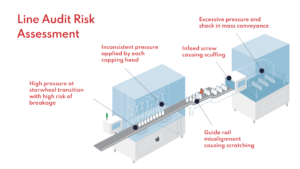
Leveraging container twins as part of line audit risk assessments.
Troubleshooting & investigations
- When damage and defect issues do arise, investigations are accelerated by access to real-time force and motion data directly from the container’s perspective.
- See not only where on the line pressure and shock events occur, but get real-time visualizations of exactly where on the container forces are impacting the product.
- Quickly identify the root cause of breakage, chips, scratching, and other glass defects and verify that the issue has been resolved on-the-spot.
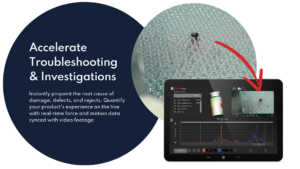
Improve yield & reliability
- Protect your products, reduce loss and minimize the risk of supply chain interruptions with instant insight into problematic areas on the line.
- By reducing damage, defects, and improper equipment setup, line reliability and yield can be improved.
To see how one pharmaceutical manufacturer achieved a 30X reduction in rejects, download the case-study here.
Tech transfer
With the CMC lifecycle growing more complex, the digitalization of knowledge is crucial. SmartSkin’s quantitative container handling data provides:
- Process-specific knowledge and an improved understanding of line and equipment performance
- Digital container handling records of line performance at each stage of the tech transfer process, from early-stage process design through process qualification and continuous process verification (CPV).
- With a better understanding exactly what containers experience in the process, knowledge transfer is accelerated, risks are better understood and mitigated, and technology transfer processes are enhanced supporting clinical to commercial expansion.

Regulatory & compliance
With routine use, the data collected by SmartSkin’s container twin technology can help pharma manufacturers meet regulatory benchmarks during audits, facilitate compliance with industry standards and support manufacturers through quality investigations.
Not only are you able to proactively identify and resolve issues to maintain high-quality standards and prevent production challenges, but historical data can also demonstrate to quality teams and regulators that the process was in control in the event of downstream glass damage and product issues.
FAT/SAT activities
During the critical FAT/SAT stages of new equipment commissioning, SmartSkin can help you meet operational requirements before full-scale production begins.
- Identify container handling issues before equipment ships so suppliers can address problems and resolve them while the equipment is still at the factory.
- Capture baseline datasets to re-assess equipment performance at SAT and ensure that all components continue to perform as intended.
- Use the technology to quickly resolve issues that arise during SAT and commissioning to keep scheduling and costs on track.
- Establish optimal baselines so line performance and yield can be maintained after routine changeovers and maintenance.
- Use the data to monitor for drift and establish preventative maintenance schedules.
Improve seal assurance strategy
A single off-spec capping head can put 5-12% of your products at risk. Assess the force applied by each capper head and monitor seal tightness after crimping to improve your seal assurance strategy and protect your drug products.
Our specialty Seal Assurance Solution allows you to specifically measure top load and seal tightness for vials and cartridges to help you mitigate quality and contamination issues and improve your container closure integrity strategy.
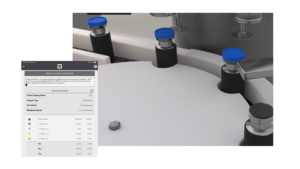
SmartSkin’s Seal Assurance solution to ensure container closure integrity.
Learn more
By identifying potential issues early with SmartSkin’s container twin technology, manufacturers can address damage and defect issues before they become problematic and ensure optimal line performance across all filling and packaging processes and throughout the equipment’s lifecycle.
To learn more about our solutions, join us at our upcoming webinar:
Leveraging Container Twin Technology in Pharmaceutical Manufacturing
Register here to join us for the live virtual session.
To learn more about the technology and our suite of solutions tailored specifically for pharmaceutical manufacturers, visit us at smartskintech.com/pharma

