smart–skin
Quantify your containers’ experience on the production line.
Get an inside look at your manufacturing line – from your containers’ perspective.
smart–skin
Quantify your containers’ experience on the production line.
Get an inside look at your manufacturing line – from your containers’ perspective.
Industries served
Purpose-built solutions to help you protect your products.
DIAGNOSE. ANALYZE. OPTIMIZE.
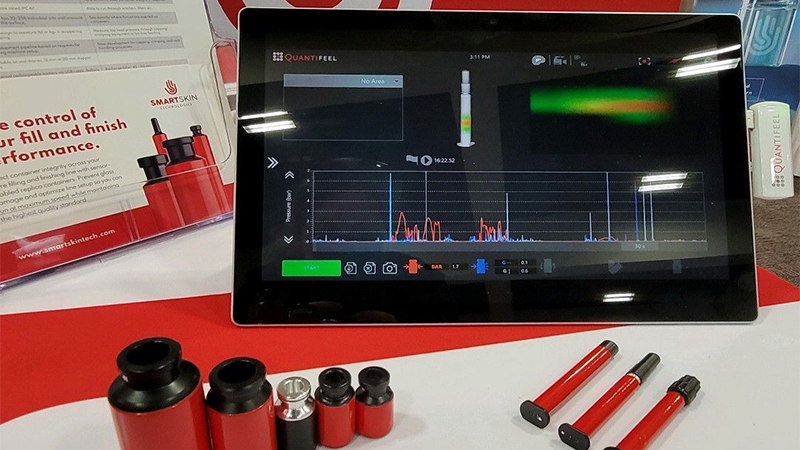
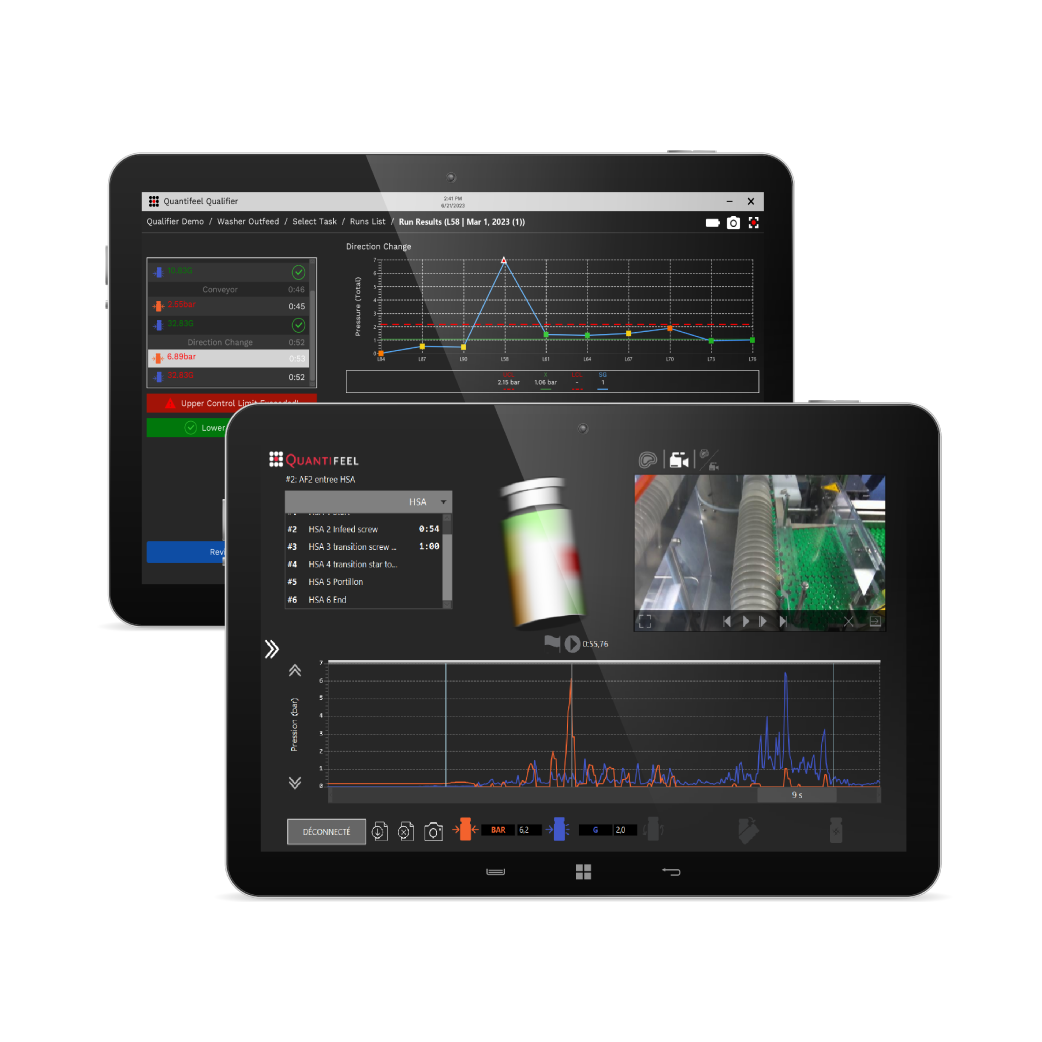
Data-driven analysis of forces impacting your products.
SmartSkin’s solutions combine innovative drone sensors and data analytics to provide pharmaceutical and beverage manufacturers with actionable data that helps them improve productivity and save valuable time.
Apply data insights to detect challenges, resolve inefficiencies, and elevate line performance.
Solutions
Technology built for your environment.
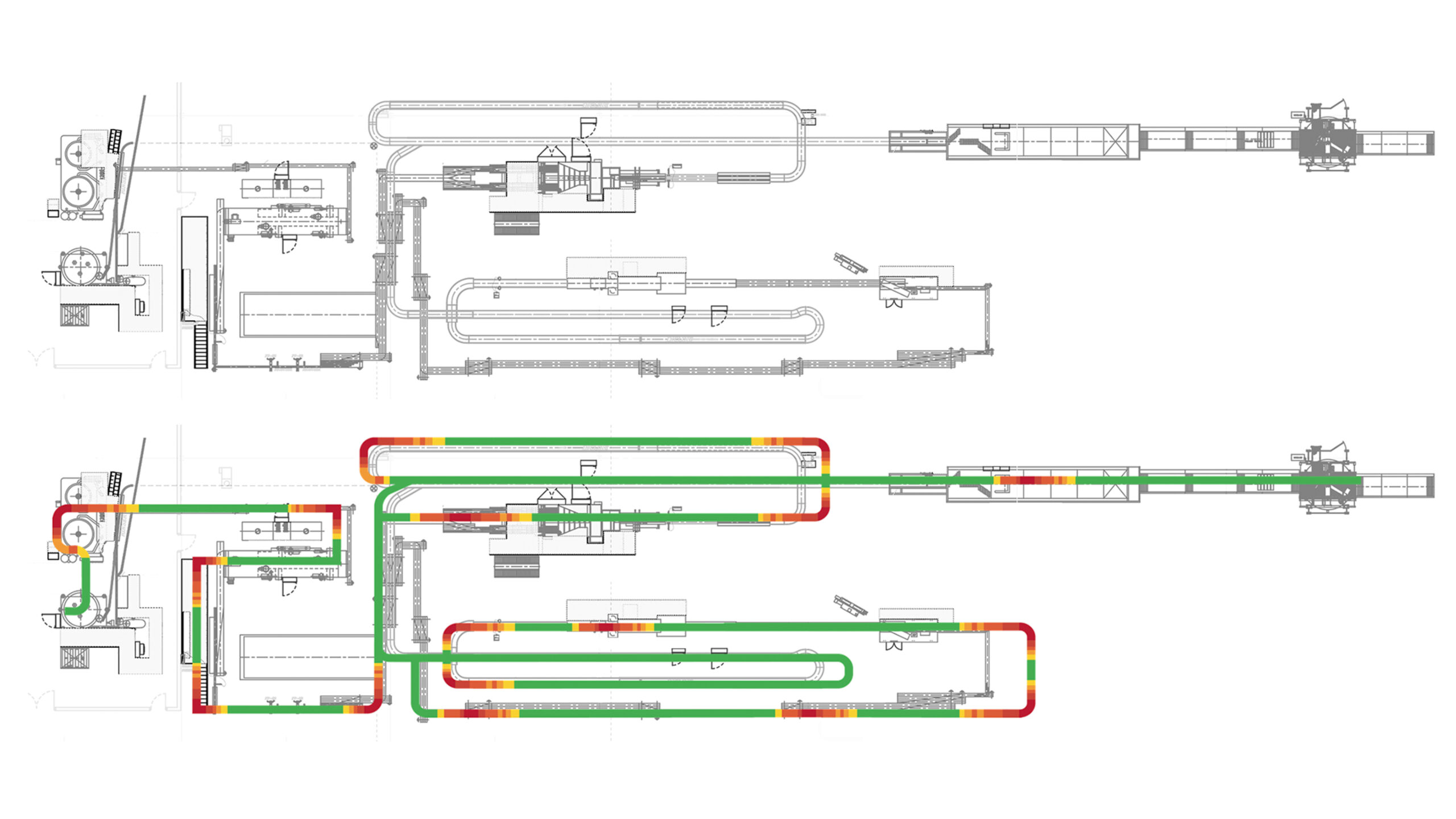
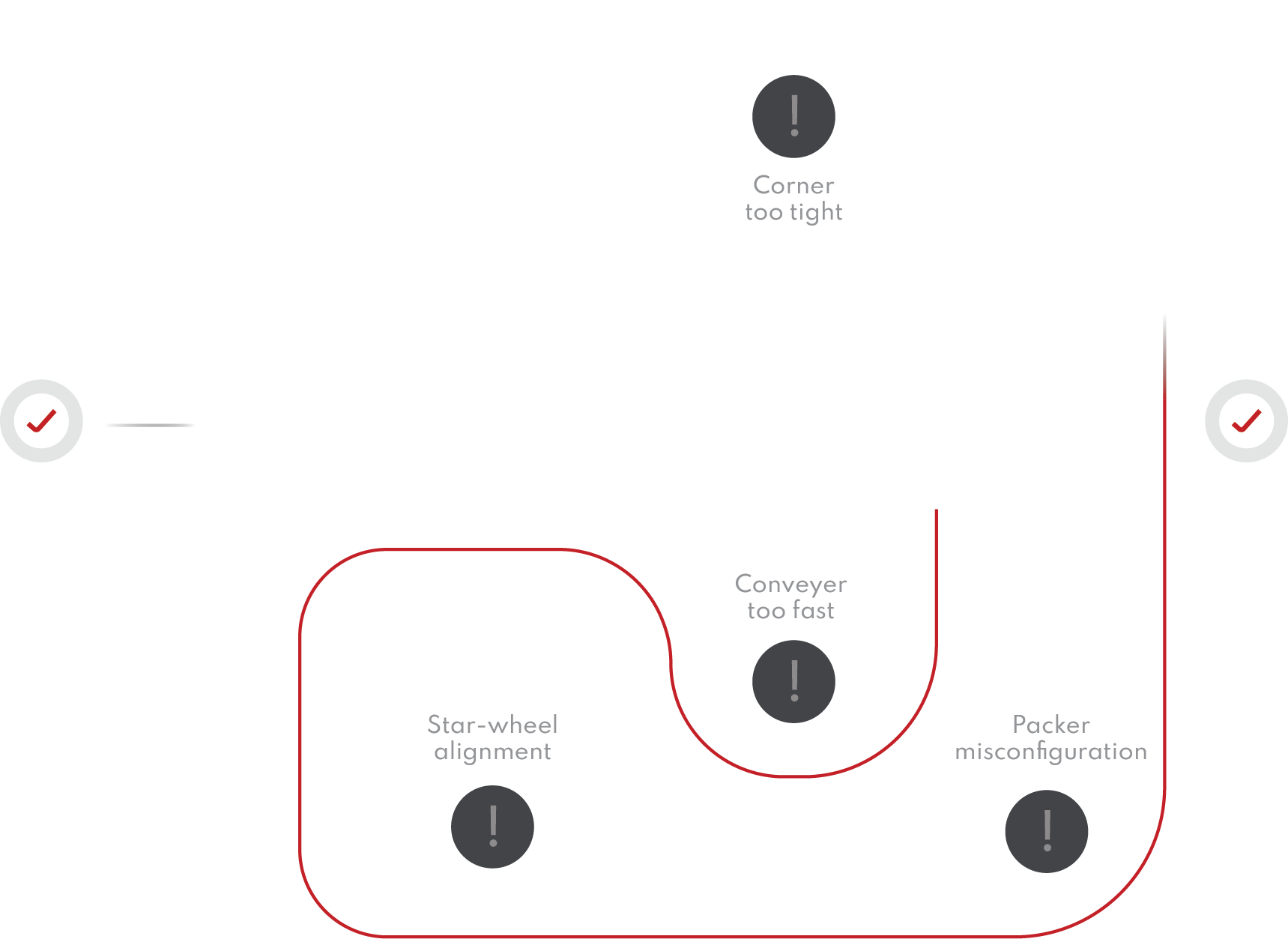
Get a complete overview of your line’s container handling performance. Quickly and easily pinpoint problem areas and present information in a powerful and accessible way.
Whether you want to see your historical line performance for better managerial decisions, compare your line to a generated industry standard, diagnose issues within machines that are otherwise unknown, or improve your overall product quality, we can get you started.
Global Presence
FROM OUR CUSTOMERS
“The damage that was done from an incident last night, without SmartSkin, would’ve taken approx. 2 weeks to fix, and costed between $200,000 to $300,000, so the ROI is there, absolutely.”
“With SmartSkin we’re able to reduce glass defects, correct unexpected line behaviour across our fill-finish lines and enhance our team’s process knowledge and mastery.”
Schedule a Demo
Solve your container handling issues today.
Toll-Free: (855) 210-9006
In the news
Most of us in the beverage industry are also consumers, and seeing damaged cans for sale on [...]
SmartSkin sensor drones return information in real-time to show where defects might be occurring during the filling [...]
From protecting the integrity of vaccine bottles during the filling and packaging process, to helping one of [...]
SmartSkin Technologies has appointed Marc Vermette as its new Chief Customer & Commercial Officer. This pivotal addition [...]
In our previous discussion about the "Exploring the domain of Quality Can Seaming: Ensuring the Perfect Seal [...]
For more than forty years, I've been deeply immersed in the world of can seaming, working across [...]